
By: Bud Bromley
September 28, 2022
Earth’s oceans can easily absorb all known and estimated carbon if that carbon were burned and CO2 emitted to the air. Ocean is an infinite sink for carbon.
This “infinite CO2 sink” is due to several factors, including importantly these two: (a) bicarbonate ion (HCO3–) is over 90% of the ionized CO2 gas in sea water. The several elemental ionic species in normal sea water which readily combine with bicarbonate ion (to form molecules or ionic pairs) are more abundant in seawater than bicarbonate ions, and (b), both CO2 gas and its ionic species as well as the several elemental ions that combine CO2’s ionic species are highly soluble in sea water under normal ocean conditions. For example, the amount of calcium Ca2+ ions in only the top ~200 meters of ocean is more than 3 times the amount of bicarbonate ions in the same surrounding seawater. But calcium is only one example; other elemental ions and molecular ions in seawater also combine with bicarbonate ions.
For example, the buffering capacity of pure water which has been saturated with dissolved calcium carbonate (i.e., CaCO3, dissolved calcite, aragonite, limestone) at standard temperature and pressure has about 100 times more buffering capacity than pure water. Buffering capacity is the ability to resist pH change, to resist pH change from addition of acid or base. Thus, we can measure “acid rain” with a pH slightly less than 7, but that “acid rain” does not result in acid ocean, lakes or rivers.
The “infinite CO2 sink” of ocean is caused by the different relative abundances of various elements on Earth. How much elemental calcium, potassium, sodium, carbon and so forth are on and in Earth’s oceans and land? Elements in rock are weathered by wind and water and become dissolved ionic content in water and eventually in ocean. So, for example, calcium is more abundant on Earth than carbon. Thus, calcium ions are more abundant in seawater than bicarbonate ions, calcium being only one example of ions that combine or pair with bicarbonate ions. Ionic charge balance in water can be temporarily and locally perturbed, but balance will be restored. For every bicarbonate ion HCO3– (a proton donor) there must be a local, offsetting ion (a proton acceptor). Ocean is in continuous contact with solid minerals (from weathering stone, seafloor, and biological organisms) which are soluble in ocean water, resulting mostly in alkalinity (abundant proton acceptors). In fact, the nefarious acidity made infamous by UN IPCC et al is weak acidity (i.e., a weak proton donor compared to a strong acid like nitric acid). The effect of the infamous but scarce carbonic acid (H2CO3) increases the buffering capacity of ocean so that it does not become too alkaline. Highly alkaline ocean would be as dangerous to life as acidic ocean. Caustic soda (sodium hydroxide) is highly alkaline 14 pH that you may use to dissolve protein, hair, gunk etc. in your clog sinks. In summary, ocean can never become acidic by CO2 from burning all known carbon on Earth. Ocean is an infinite sink for Earth’s carbon, at least all carbon we know about and can estimate. Of course, this is the opposite of the false narrative from AGW proponents.
We may speculate that maybe we are hit by a giant carbon asteroid. Or maybe Earth’s core is loaded with carbon and a giant fissure opens one day? But the probability of these speculations occurring is higher than the probability of ocean becoming acidic due to atmospheric CO2 from fossil fuels. To change our alkaline oceans to acid, a very, very large amount of a strong acid (e.g. sulfuric acid or nitric acid) would be required, not the weak carbonic acid that is formed from the tiny 0.0089% increase in atmospheric CO2 that has occurred during the last 50 years (1970-2020).
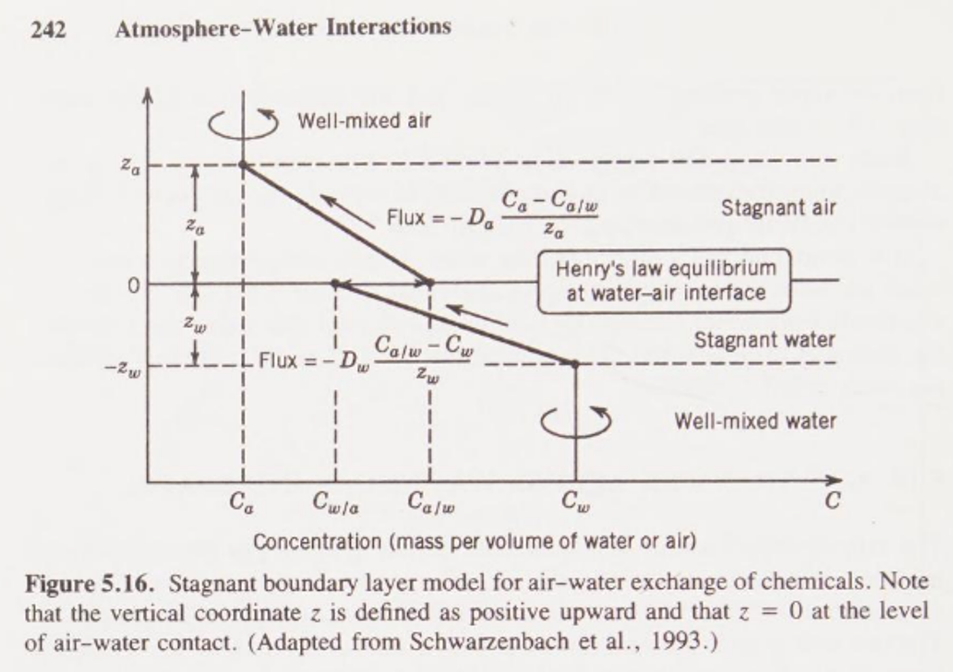
Note the higher abundance in seawater of calcium ion Ca2+ compared to bicarbonate ion HCO3–

It is not possible to calculate an average pH of ocean. “Oceanic pH varies naturally with latitude and ocean depth across Earth. Hence, no single value exists to define oceanic pH. For instance, the pH of surface waters in the western Pacific Ocean varies from around 7.8 to 8.5 between 60°N and 60°S. Although significant pH variations can occur both above and below this range, ocean water is generally characterized as alkaline. And because pH units of measure fall on a logarithmic scale (each pH unit change requires an order of magnitude change in the activity of H+ )… The ocean acidification hypothesis also ignores the presence of vast amounts of dissolved calcium in the ocean: the upper 200 m of ocean water contains enough dissolved calcium to bind all anthropogenic CO2 as precipitated calcium carbonate (in the ocean) without affecting the ocean’s pH (Jaworowski et al., 1992a; Segalstad, 1996; 1998). The ocean acidification hypothesis also ignores or downplays other oceanic buffers (pH stabilizing reactions), the thermodynamic stability of solid calcium carbonate in ocean water, and photosynthesis by marine biological systems.” ~ Tom V. Segalstad, PhD. (2014) Some Thoughts on Ocean Chemistry, (Chapter 6.3.1.2) Contributed by Tom V. Segalstad Associate Professor of Geochemistry (Resource- and Environmental Geology) University of Oslo, Norway. https://www.researchgate.net/publication/304797201_Some_thoughts_on_ocean_chemistry_Chapter_6312
It does not matter how much CO2 humans emit into the air. Humans have not and cannot change global atmospheric CO2 concentration except very temporarily and locally. The relative partition of CO2 gas between ocean surface and air is determined by Henry’s Law, the Law of Mass Action, Fick’s Law and Graham’s Law. Ocean surface is 71% of Earth’s surface. CO2 concentration in air will always adjust to find an equilibrium, such as 400 ppm, always a variable of the sea surface temperature, but CO2 is not dependent on the amount of CO2 gas in the air. CO2 gas (or any gas) diffusion into sea surface (or any liquid) is inversely proportion to the temperature of the surface, while the flux or rate of diffusion is directly proportional to the area of surface at that temperature. The flux rate (i.e., moles of CO2 gas diffusing into or out of a square kilometer of sea surface per day) is a direct function of the number of square kilometers of sea surface at a given temperature. The concentration of CO2 gas in air cannot change sea surface temperature. Sea surface temperature changes CO2 gas concentration in the air above the surface, then that air is mixed by winds and other forces in the atmosphere. For example, less cloud cover, resulting more insolation, over a square kilometer of sea surface results in higher CO2 flux rate or emission into air. And vice versa. CO2 is always being both emitted and absorbed simultaneously from sea surface, but the rates of emission and absorption are not equal.
Net global CO2 concentration in air today is the same as it would be if humans never existed. The Henry’s Law partition ratio of CO2 gas (or any other gas) between water and air is an intensive property of matter, like boiling point or specific gravity; increasing or decreasing the amount of the gas in air does not change the Henry’s Law partition ratio for a given temperature.
Bert Bolin, PhD, Swedish meteorologist and the first chairman of the United Nations Intergovernmental Panel on Climate Change (IPCC), from 1988 to 1997, correctly stated in Bolin & Eriksson, 1959: “First we see that if the partial pressure of CO2 varies and the hydrogen ion concentration were kept constant, the relative changes would be the same in the sea as in the atmosphere. As the total amount of CO2 in the sea is about 50 times that in the air, practically all excess CO2 delivered to the atmosphere would be taken up by the sea when equilibrium has been established.” The hydrogen ion concentration referred to is pH of ocean surface, which is highly buffered in both directions by mineral ions and molecular ions dissolved in ocean surface, including the dissolved ions of CO2.
In local and temporary conditions, pH and alkalinity as well as CO2 emission and absorption fluxes can change significantly, swinging one way and then reversing, diurnally (within a day), and then rebalance to trend. For example, during daylight, plankton in ocean surface absorb large amounts of CO2 for their photosynthesis. Then during night they emit large amounts of oxygen from their respiration. Their absorption of CO2 from sea water during daylight temporarily depletes CO2 concentration in the sea surface, a perturbation to the Henry’s Law ratio, causing a relative increase in CO2 absorption flux from air into ocean surface to rebalance Henry’s Law partition ratio. Meanwhile, it is day, insolation, and sea surface is warming, thus CO2 emission flux from sea surface to air is increasing. Plankton, by depleting surface CO2 have caused the Henry’s ratio to adjust. While CO2 emission flux is increasing due to warming, CO2 absorption flux increases relatively more until sunset. CO2 absorption by plankton is faster than the net increase in CO2 absorption flux into sea surface from air, thus sea surface becomes temporarily progressively more depleted of aqueous CO2 gas during the day. During daylight, pH and alkalinity increase. That night, without sunlight (except slightly at high latitudes), CO2 absorption by plankton stops, CO2 re-saturates up to the Henry’s partition ratio for the surface temperature, which is usually a cooler surface resulting in relatively higher CO2 concentration in ocean surface during night, and at the same time pH and alkalinity both decrease slightly due to more carbonic acid in the surface water. Winds, currents, storms and salinity cause similar temporary and local perturbations. It is a multi-dimensional dynamic equilibrium.
The increase in Mauna Loa-measured global CO2 concentration over the last 50 years (1970 – 2020) has been about 89 ppm. That is, the cumulative increase in CO2 concentration due to all CO2 sources and sinks, natural and human, over the last 50 years is less than 0.0089% of the atmosphere. Your exhaled breath is about 4% CO2.



