By Bud Bromley | August 18, 2021
In physical chemistry, Henry’s Law is one of the gas laws. It defines the solubility of a gas within a liquid which is in contact with the gas. It was formulated in the early 19th century by the English chemist William Henry who described the results of his experiments. In the 21st century, Henry’s Law is the foundation science for multi-billion per year industries, for example the multi-billion dollar per year scientific instruments business of gas chromatography. Henry’s Law also explains why the theory of human-caused global warming /climate change is not even plausible science and should be shunned by knowledgeable people.
Henry’s Law is specifically limited to a phase state equilibrium reaction of a gas and that same gas which is in contact with a liquid solvent, for example CO2 gas in the atmosphere which is in contact with ocean water. Henry’s law only applies to low concentrations of a gas in the mixed gas phase and in the liquid phase. Henry’s Law does not apply to the series of carbonate chemistry reactions occurring in ocean water after the CO2 gas reacts with water and hydrates and disassociates into ions. CO2 gas in atmosphere and in ocean surface satisfies these conditions.
Henry’s Law is dominantly dependent on the temperature at the interface of the gas with the liquid. Rearranging Henry’s Law as d(ln(kH))/d(1/T) defines the temperature dependence parameter in Henry’s Law partition co-efficient, where kH is the Henry’s Law constant and T is temperature in Kelvin. Henry’s Law is explained on the website of the U.S. National Institutes of Standards and Technology. https://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Units=SI&Mask=10#Solubility
“The chemistry of carbon dioxide is quite complex, but it boils down to reactions as in Eq. (I). In the first step, CO2 of the atmosphere dissolves into the ocean CO2(g)⇌CO2(aq). (I)
In water the CO2 molecules combine with water molecules to form H2CO3 , and this reaction can be written as CO2(aq)+H2O(l )⇌H2CO3(aq). (II)
Here the ratio of the two concentrations of Eq. (I) is given by Henry’s Constant, that depends on the temperature (see Table 6-7 of Lide and Frederikse (1974)), ?h(?)= [CO2(g)] / [CO2(aq)]. (3)” (Stallinga, P. (2018). Carbon Dioxide and Ocean Acidification. European Scientific Journal, ESJ, 14(18), 476. https://doi.org/10.19044/esj.2018.v14n18p476 )
Since the concentration of net global CO2 gas concentration is routinely measured, then Henry’s Law can be used to calculate the concentration of aqueous CO2 gas in ocean surface water.
If net global CO2 concentration in air is 400 ppmv, then, per Henry’s Law KH, at STP, 400 x 10-6 atm. [CO2] = P/KH = 4.00 x 10-4 atm/29.41 atm M-1 = 1.36 x 10-5 M (moles of aqueous CO2 gas per liter of surface ocean water.)
Theoretically, this concentration of aqueous CO2 gas then can be used to roughly calculate the concentration of the carbonates in the series of acid-base reactions in ocean water which occur subsequent to the Henry’s Law phase-state equilibrium reaction, but there are many different equilibrium constants and ocean and atmosphere are very dynamic.
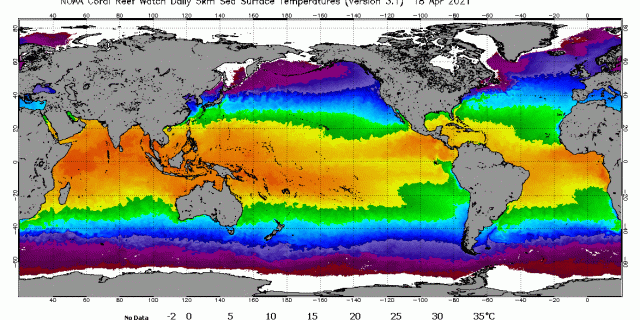
It is incorrect to use average global temperature in Henry’s law calculations, unless that temperature average is weighted by surface area of ocean at a given temperature. However I have not found that information in the scientific literature. Use of global average temperature results in unexplained errors in CO2 concentration. For example, global average ocean temperature is about 17 C, which in the Henry’s coefficient would define ocean as absorbing CO2, which leads to the question: how could global average CO2 concentration be increasing? But the average temperature of the tropical ocean surface is above 26 C year round, which implies tropical ocean surface is emitting CO2 gas year round. The Henry’s Law constant for CO2 and ocean surface water is different at 17 C and 26 C. The Henry’s constant is not constant for different temperatures, which means the ratio of CO2 gas in air versus CO2 gas in ocean water is different at different temperatures. Thus Henry’s Law constants in chemistry text books vary by temperature. A few examples of common errors in the climatology literature are provided below.
One example is in the following graph, the carefully measured net global CO2 concentration trend (measured at Mauna Loa) versus the RSS satellite-measured global mean surface temperature. The two trends are diverging which by itself immediately falsifies anthropogenic global warming theory. Temperature, density, solubility, pH, specific volume, specific entropy, thermal conductivity, thermal expansion, compressibility are intensive properties in thermodynamics; an intensive property depends only on the type of matter and not on the amount of matter and may vary from place to place within the system at any moment. The average temperature of the earth and the average temperature of earth’s troposphere provide no useful information regarding CO2 flux nor CO2 concentration change nor Henry’s Law and result in errors in calculating CO2 concentration and flux. For example, using Henry’s Law and average ocean temperature of 17 C results in an average global CO2 concentration of about 312 ppmv, which is obviously a large error based on Mauna-Loa-observed net global average CO2 concentraton of about 417 ppmv.

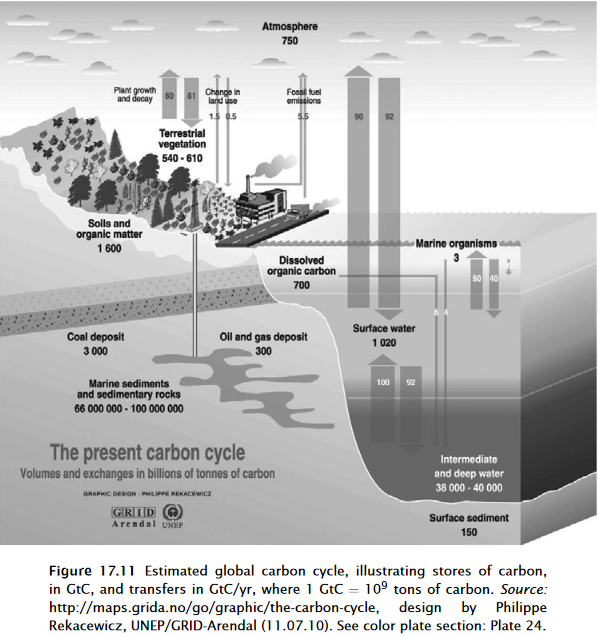
Henry’s Law and its partition ratio (co-efficient) for CO2 apply only to this equilibrium reaction: CO2(g)⇌CO2(aq). Henry’s Law does not apply to the hydration reaction of CO2(aq) nor to the several carbonate ion reactions that follow. The ratio of the two concentrations of this first phase state reaction [CO2(g)]⇌[CO2(aq)] is given by the Henry’s co-efficient which depends on temperature (see for example Table 6-7 of Lide and Frederikse (1974), or the Handbook of Chemistry and Physics found in almost all chemistry labs.) In this initial reaction, CO2(g) has not disassociated. Henry’s Law determines the exchange of CO2(g) between the estimated 1000 gigatonnes CO2(g) in ocean surface and the estimated 700 gigatonnes of CO2(g) in the atmosphere; the exchange is estimated as two 90 gigatonnes CO2 per year fluxes in opposite directions, into air and into ocean surface. For comparison humans are estimated to emit into air about 8 gigatonnes of CO2 per year, which is immediately, chaotically mixed with the two 90 gigatonne fluxes into the air and ocean CO2 reservoirs which are about 10 times larger.

In most climatology literature, aqueous CO2 gas is bundled together and summed with carbonic acid, bicarbonate and carbonate anions in the several initial carbonate chemistry reactions and is usually quantified within the total in millimol/kg-of-solution dissolved inorganic carbon (DIC). But each of these carbon moieties vary based on multiple factors, mainly temperature of the ocean surface, salinity, alkalinity, and CO2 concentration in air immediately above the ocean surface. Consequently, the important Henry’s equilibrium is ignored or confused in usual climate literature resulting in significant, unexplained errors. For example, DIC is directly proportional to CO2(g) ppm in air, but DIC is inversely proportional to ocean surface temperature. If CO2(g) is constant at 400 ppm, then DIC is inversely proportional to ocean temperature while increasing temperature is directly proportional to increasing pH. Clearly it is mistaken to bundle these co-dependent offsetting chemical entities into a single one-dimensional hypothetical reactant. http://www.molecularmodels.eu/Ocean-CO2.pdf
“The analysis of dissolved CO2 in water is an important basis for the assessment of the role of surface waters in the global carbon cycle (Raymond et al., 2013). Indirect methods like calculating CO2 from other parameters like alkalinity and pH (Lewis and Wallace, 1998; Robbins et al., 2010) are affected by considerable random and systematic errors (Golub et al., 2017) caused for example by dissolved organic carbon, which may result in significant overestimation of the CO2 partial pressure (pCO2) (Abril et al., 2015), or by pH measurement errors (Liu et al., 2020). Thus, direct measurement of CO2 is highly recommended, particularly in soft waters.” Koschorreck, M., Prairie, Y. T., Kim, J., and Marcé, R.: Technical note: CO2 is not like CH4 – limits of and corrections to the headspace method to analyse pCO2 in fresh water, Biogeosciences, 18, 1619–1627, https://doi.org/10.5194/bg-18-1619-2021, 2021.
“Measurements of the atmospheric CO2 concentration indicate that it has been increasing at a rate about 50% of that which is expected from all industrial CO2 emissions. The oceans have been considered to be a major sink for CO2. Hence the improved knowledge of the net transport flux across the air–sea interface is important for understanding the fate of this important greenhouse gas emitted into the earth’s atmosphere (1–5).” … “Sources of Errors. The flux estimates are subject to errors from the following five independent sources: (i) the gas transfer coefficients, (ii) the wind speed variability, (iii) the normalization of observations to the reference year of 1990, (iv) the interpolation of limited observations, and (v) skin temperature effect. “The estimated flux values, which range from 0.60 to 1.34 Gt-Czyr21, depend on the choice of sea–air CO2 gas transfer formulations. Hence the error is of a systematic nature and may be reduced if the gas transfer coefficient is better understood in the future.” Taro Takahashi et.al. Global air-sea flux of CO2: An estimate based on measurements of sea–air pCO2 difference. https://www.pnas.org/content/pnas/94/16/8292.full.pdf
The enormous excess of aqueous CO2(g) in ocean is controlling the stoichiometry. Meanwhile the activities of carbonic acid and carbonate ions are constrained, being suppressed by the 1000 times excess of calcium and silicon ions with respect to carbonate ions as well as alkalinity in the ocean buffering systems.
The alkaline ocean and buffers for carbonate ions allow seawater to dissolve and react with huge amounts of aqueous CO2 gas, which in turn causes absorption of additional CO2 gas from atmosphere into the ocean surface. To calculate the true equilibrium value of CO2 gas in the air and aqueous CO2 in ocean surface, all stoichiometry mass balances and kinetics of all of the carbonate chemical reactions must be simultaneously computed, accounting for the equilibrium constant of each reaction, and each of these reactions in turn depend on temperature, alkalinity, and salinity. These are complicated simultaneous differential equations.
In ocean water, CO2 molecules hydrate in seconds to form H2CO3, but this does not mean the CO2(g)⇌CO2(aq) equilibrium can be ignored as usually done in climatology literature. Evidence of over 1000 gigatonnes of aqueous CO2 gas in ocean surface is definitive. Also the hydration reaction aqueous CO2(g) + H2O(l) ⇌ H2CO3(aq) is more complicated than it is usually represented in climatology literature. Combining the CO2(g)⇌CO2(aq) reaction with the CO2 hydration reaction and calling it HCO3* or H2CO3*, which are non-existing hypothetical entities, leads to stoichiometric errors in the carbonate chemistry reactions as well as in the CO2(g)⇌CO2(aq) equilibrium reaction. For example in “Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium” by Timothy J Lueker Andrew G Dickson Charles D Keeling. https://www.sciencedirect.com/science/article/abs/pii/S0304420300000220. or https://doi.org/10.1016/S0304-4203(00)00022-0 The kinetics and stoichiometry of hydration are more complicated and the chemistry and kinetics of CO2(g)⇌CO2(aq) are omitted entirely.
The hydration equilibrium constant at 25°C is called Kh, which in the case of carbonic acid is [H2CO3]/[CO2] ≈ 1.2×10−3 in seawater. Hence, the majority of the carbon dioxide in sea water is not converted into carbonic acid, remaining as CO2 molecules. Alan L. Soli, Robert H. Byrne, CO2 system hydration and dehydration kinetics and the equilibrium CO2/H2CO3 ratio in aqueous NaCl solution, Marine Chemistry, Volume 78, Issues 2–3, 2002, Pages 65-73, ISSN 0304-4203, https://doi.org/10.1016/S0304-4203(02)00010-5. https://www.sciencedirect.com/science/article/pii/S0304420302000105

Source: Stumm and Morgan. Aquatic Chemistry. P210.
This short video explains the Henry’s Law equilibrium equation. Note also the professor’s comment about ammonia being “scrubbed” by water since CO2 is “scrubbed” from the air by water in ocean surface in the same manner. https://archive.org/details/ucberkeley_webcast_-ziliLAdom4

As already mentioned, the Henry’s equilibrium “constant” or co-efficient for CO2(g) is not constant with respect to temperature. Also, the equilibrium “constants” for 3 other reactants and products in the initial carbonate chemistry series are not “constant” with respect to temperature. Figure 2.2 above from Morel illustrates significant variation in the equilibrium constants with respect to standard temperature (25 C). Clearly, bundling these offsetting widely varying reactants and products together in a bundled hypothetical reaction will lead to errors.
“The Henry’s constant typically increases with temperature at low temperatures, reaches a maximum, and then decreases at higher temperatures. The temperature at which the maximum occurs depends on the specific solute-solvent pair.”…”It is important to recognize that the Henry’s “constant” is a strong, nonlinear function of temperature. For accurate design, it is preferable to have temperature-dependent data for Hi(T).”.. Additional pitfalls include failing to distinguish between the “solubility” and the “volatility” form of Henry’s law, failing to consider the implications of liquid-phase solute partitioning, and failing to be careful about units of measure, especially dimensionless units.” Harvey, A. and Smith, F. (2007), Avoid Common Pitfalls when using Henry’s Law, Chemical Engineering Progress, [online], https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=50449 (Accessed August 14, 2021).
Example papers of observed temperature-dependent CO2 emission or absorption or fugacity from ocean surface:
“Based on these observations, 72% of the increase in fCO2sea in Cariaco Basin between 1996 and 2008 can be attributed to an increasing temperature trend of surface waters, making this the primary factor controlling fugacity at this location.” Astor, Y. M.; Lorenzoni, Laura; Thunell, R.; Varela, R.; Muller-Karger, Frank E.; Troccoli, L.; Taylor, G. T.; Scranton, M. I.; Tappa, E.; and Rueda, Digna, “Interannual Variability in Sea Surface Temperature and Fco2 Changes in the Cariaco Basin” (2013). Marine Science Faculty Publications. 1057.
https://scholarcommons.usf.edu/msc_facpub/1057 . https://doi.org/10.1016/j.dsr2.2013.01.002
Note that the discussion and interpretations in the above paper attempt to support AGW. But the data in the paper support the case which is presented here. There are many papers like this from geographies around the world.
“Small changes in the largest marine carbon pool, the dissolved inorganic carbon pool, can have a profound impact on the carbon dioxide (CO2) flux between the ocean and the atmosphere, and the feedback of this flux to climate.” Irina I. Pipko, et.al. The spatial and interannual dynamics of the surface water carbonate system and air–sea CO2 fluxes in the outer shelf and slope of the Eurasian Arctic Ocean. Ocean Sci., 13, 997–1016, 2017
https://doi.org/10.5194/os-13-997-2017
“On the one hand, the hypothesis of Henry’s Law tells us that CO2 variations in the atmosphere are the result of temperature changes. We had already shown that for historical long-term data Henry’s Law is correct [ 12 23 ], as well as for periodic data of a specific meteorological station, De Bilt (Netherlands) [ 17 ].” … “Analyzing the contemporary data of Figure 3, we can conclude that Henry’s Law can readily explain this: every time warm water appears at the surface of the oceans, a huge amount of CO2 is released into the atmosphere (or less is absorbed than could be absorbed by cold waters), and in La Niña years when cold water reaches the surface, more CO2 is taken back to the oceans. It is even consistent with the value obtained on basis of thousands-of-years historical data (10 ppm/K [ 12 ]).” Analysis of Temporal Signals of Climate. Peter Stallinga, Igor Khmelinskii. FCT and CEOT, University of the Algarve, Faro, Portugal. DOI: 10.4236/ns.2018.1010037 . https://www.scirp.org/pdf/NS_2018101510264849.pdf
“The biogeochemical cycling of carbon between its sources and sinks determines the rate of increase in atmospheric CO2 concentrations. The observed increase in atmospheric CO2 content is less than the estimated release from fossil fuel consumption and deforestation. This discrepancy can be explained by interactions between the atmosphere and other global carbon reservoirs such as the oceans, and the terrestrial biosphere including soils. Undoubtedly, the oceans have been the most important sinks for CO2 produced by man.” … “The instability of current models to estimate accurately oceanic uptake of CO2 creates one of the key uncertainties in predictions of atmospheric CO2 increases and climate responses over the next 100 to 200 years.” Peng, T H, Post, W M, DeAngelis, D L, Dale, V H, and Farrell, M P. Atmospheric carbon dioxide and the global carbon cycle: The key uncertainties. United States: N. p., 1987. Web. ACS Publications.
Note that the discussion and interpretations in the paper immediately above attempt to support AGW. But the data in the paper support the case which is presented here. There are many papers like this from all around the world.
“There is a decline, or a negative trend, in the air-sea pCO2 gradient of 23 μatm over the decade, which can be explained by a cooling of 1.3 °C over the same period.” Shadwick, E. H. et al., Air-Sea CO2 fluxes on the Scotian Shelf: seasonal to multi-annual variability. Biogeosciences, Volume 7, Issue 11, 2010, pp.3851-3867. November 2010. DOI:10.5194/bg-7-3851-2010. https://bg.copernicus.org/articles/7/3851/2010/bg-7-3851-2010.pdf
The rate limiting step for re-equilibrating the partial pressure of aqueous CO2 gas in ocean surface is the migration time for CO2 gas in the ocean surface water. This is both vertical and horizontal migration and there are many variables.
Changes in atmospheric CO2 concentration are observed to lag (occur after) variations in earth’s surface temperature by 6 to 9 months and longer. The papers by Stallinga and Khmelinskii above discuss this in detail using the example of Pinatubo volcano eruption in 1991, which, after the volcano injection of CO2, the net global CO2 concentration returns to the ongoing long term rate of change of CO2 concentration in air with respect to time (dCO2/dt) and then returns to the Henry’s partition ratio which existed prior to the perturbation in ocean surface temperature which was caused by the global belt of high altitude clouds and ash; no permanent or long term offset of CO2 concentration or slope occurred.
“It is critical to note that the sea must heat before the level of atmospheric CO2 can rise – not the other way around. This is important because it proves that increasing levels of anthropogenic produced CO2 cannot cause the sea to heat initially – whether that additional CO2 causes the global surface temperatures to rise or not. Satellite records shows there is a recorded increase in sea surface temperatures over recent years which basic science, shows must be causative of increased levels of CO2 in the atmosphere. CO2 is a heavy gas (1.5 times air) and remains in close contact with the sea surface. This permits rapid stoichiometric balancing of sea and atmospheric CO2 during season changes.” https://bosmin.com/SeaChange.pdf
“The rate of mass transfer from pure CO2 effluent discharged in the deep ocean depends strongly on the solubility of CO2 in seawater. This thermodynamic study derives solubility relationships for both gas- and liquid-phase CO2 in seawater. It is determined that, for CO2 gas, solubility depends on both temperature and pressure and, as a consequence, solubility increases sharply with depth in the ocean.” H.Teng1S.M.Masutani1 C.M.Kinoshita1 G.C.Nihous2, Solubility of CO2 in the ocean and its effect on CO2. https://doi.org/10.1016/0196-8904(95)00294-4
When ocean surface exceeds 25.6 C it out-gases CO2 gas into the air from ocean surface. Simultaneously, in another location, ocean surface which is less than 25.6 C will absorb CO2 gas into the ocean surface. Both CO2 absorption and emission are almost immediate if the aqueous CO2 gas is present at the ocean surface. If the ocean surface has been warm, windy or turbulent for an extended time period, then the partial pressure aqueous CO2 gas in the surface will be lower, that is under-saturated with CO2 with respect to the Henry’s ratio for that temperature. Although the out-gassing step itself and activation energy would be sufficient, the depletion of the aqueous CO2 gas from the local surface area requires time to recover to saturation due to the resistance to migration of the CO2 in the ocean water matrix.
“Here we use global-scale atmospheric CO2 measurements, CO2 emission inventories and their full range of uncertainties to calculate changes in global CO2 sources and sinks during the past 50 years. Our mass balance analysis shows that net global carbon uptake has increased significantly by about 0.05 billion tonnes of carbon per year and that global carbon uptake doubled, from 2.4+/-0.8 to 5.0+/-0.9 billion tonnes per year, between 1960 and 2010.” https://cfc.umt.edu/research/gcel/files/Ballantyne_IncreasedCO2Uptake_Nature_2012.pdf
In other words, globally averaged, CO2 concentration in air is increasing and CO2 gas concentration in ocean surface is also increasing. The Henry’s partition ratio is re-equilibrating to a larger area of ocean surface which is above 25.6 C.
There are 2 thin layers at the gas – liquid ocean surface which slightly delay emission of CO2 gas from ocean surface into air. But this delay is not the rate limiting step for the emission flux. These thin films involve simultaneous virial kinetics, Van der Waals forces and surface tension.
The late professor Lance Endersbee demonstrated that a straight line relationship exists with near perfect correlation between sea temperature with respect to CO2 concentration, consistent with Henry’s Law when time is de-seasonalized. http://icecap.us/images/uploads/Focus_0808_endersbee.pdf

In conclusion, science observations and theory confirm that the atmospheric level of CO2 is always in balance with the sea surface temperature or else is dynamically moving through very large fluxes to re-establish that balance. In one geography, CO2 gas is being absorbed into ocean surface while in another location CO2 is being emitted into air from the ocean surface. The amount of CO2 gas emitted into air by humans (about 8 gigatonns per year) is trivially small with respect to both the existing CO2 reservoir in air (about 700 gigatonnes) and the existing CO2 reservoir in ocean surface (about 1000 gigatonnes) and it is also trivially small with respect to the two ongoing annual fluxes of CO2 (about 90 gigatonnes each) circulating between the atmospheric CO2 reservoir and ocean surface reservoir. The human emission is easily absorbed by ocean surface. But the critically important point is that the source of the CO2 is not a variable in Henry’s law equilibrium reaction. Atmospheric CO2 concentration is not affected by humans but instead is controlled by natural chemical and physical processes which control CO2 solubility in ocean water. Similarly, CO2 residence time in atmospheric is also irrelevant to this chemistry and physics. CO2 gas will always find the equilibrium concentration ratio between sea surface and air determined by Henry’s Law which is a function of sea surface temperature at that location. Globally averaged, the other variables which are functions in Henry’s Law cancel out, i.e., salinity, alkalinity, surface winds, water surface turbulence, and pressure cancel out globally, though they do have effect locally.
Hermann Harde. What Humans Contribute to Atmospheric CO2: Comparison of Carbon Cycle Models with Observations. Earth Sciences. Vol. 8, No. 3, 2019, pp. 139-159. doi: 10.11648/j.earth.20190803.13 http://www.sciencepublishinggroup.com/journal/paperinfo?journalid=161&doi=10.11648/j.earth.20190803.13%20
M. L. Salby, “Atmospheric Carbon”, Video Presentation, July 18, 2016. University College London. https://youtu.be/3q-M_uYkpT0.
M. L. Salby, “What is Really Behind the Increase of Atmospheric CO2“? Helmut-Schmidt-University Hamburg, 10. October 2018, https://youtu.be/rohF6K2avtY
M. L. Salby, “Relationship Between Greenhouse Gases and Global Temperature”, Video Presentation, April 18, 2013. Helmut-Schmidt-University Hamburg https://www.youtube.com/watch?v=2ROw_cDKwc0.
E. Berry, “Human CO2 has little effect on atmospheric CO2“, 2019. https://edberry.com/blog/climate-physics/agw-hypothesis/contradictions-to-ipccs-climate-change-theory/
Stallinga, P. (2020) Comprehensive Analytical Study of the Greenhouse Effect of the Atmosphere. Atmospheric and Climate Sciences, 10, 40-80. Full paper in pdf here: https://www.scirp.org/pdf/acs_2020011611163731.pdf
Analysis of Temporal Signals of Climate. Peter Stallinga, Igor Khmelinskii. FCT and CEOT, University of the Algarve, Faro, Portugal. DOI: 10.4236/ns.2018.1010037